Osteoarthritis (OA) describes inflammation of a joint and occurs after single or repetitive episodes of trauma. The term incorporates synovitis, capsulitis, sprain, intra-articular fractures, meniscal tears, and OA. These pathological conditions are ‘a group of overlapping distinct diseases which may have different aetiologies, but with similar biologic, morphologic, and clinical outcomes’.
Although conventional concepts of OA emphasize the direct and predominant involvement of cartilage and bone in OA development, it is increasingly recognised that the synovium has a significant effect on the central pathophysiological event of cartilage damage.
Arthramid Vet- A Novel Treatment
2.5% PAAG is a novel treatment for OA, and its clinical efficacy recorded in horses, goat and human models. 2.5% PAAG is different from other described hydrogels which have different concentrations of polyacrylamide and may contain other components as well e.g. silver ions. Furthermore, PAAG products, although often considered equal, have clear differences in composition, manufacturing and injection techniques as well as their ability to interact with surrounding tissues. Characteristics that ultimately determine the safety and effectiveness of each hydrogel.
Hydrogels are 3-dimensional, hydrophilic, polymeric networks capable of absorbing large amounts of water or biological fluids. Due to their high-water content, porosity and soft consistency, they closely simulate natural living tissue. In addition to biocompatibility, 2.5% PAAG is shown to be long-lasting and non-degradable. Histological examinations of treated OA joints, while showing a large number of infiltrating macrophages with some evidence of phagocytosis, illustrate the 2.5% PAAG is fully integrated into the synovial membrane by between 14 to 42 days post-treatment, and still present up to 2 years.
Potential Mechanisms of Action of 2.5% PAAG
Tnibar et al. (2015) investigated possible biomechanical mechanisms of action of 2.5% PAAG in OA joints, based on MRI, pathology and joint capsule elasticity investigations. Stabilization of OA lesions was seen on MRI indicating a possible protective effect of the 2.5% PAAG attributable to the high visco-supplementation. Additionally, an increase in joint capsule elasticity, caused by augmentation of the soft tissues of the joint and in particular, the synovial membrane, may reduce the overall joint capsule stiffness as shown in the following graphs;
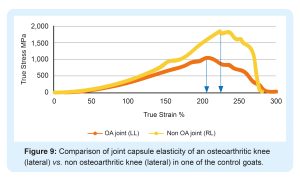
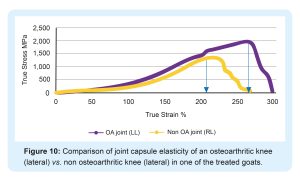
2.5% PAAG significantly increased joint capsule elasticity and alleviated lameness during the first month after treatment. This effect lasted and increased progressively until six months, with stabilization between 6 and 24 months. Success was attributed to improved load transfer capacity of the joint capsule, which in turn reduces mechanoreceptor activation and disrupting the catabolic pathways characteristic of OA.
Another recent study has shown that PAAG lubricates cartilage after mechanical injury and biochemical degradation. This was shown by a lowering of the coefficient of friction by almost 60% in the presence of PAAG hydrogel (Vishwanath et al, 2021).
Christensen (2015) showed that after injection into the joint there is formation of a stable, long-lasting sub-synovial layer of gel traversed with thin strands of connective tissue. It was uniquely demonstrated the formation of a novel synovial lining layer after integration, with no giant cells at any time.
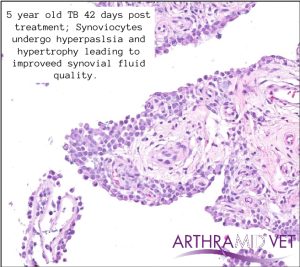
Clinical Trial Findings
These findings are supported in clinical trials in horses, where OA joints that respond to treatment of 2.5% PAAG show a greater chance of resolution of lameness, joint effusion, and reaction to passive flexion at four and six weeks. The dramatic and significant reduction in joint effusion supports the suggestion that 2.5% PAAG has a disease-modifying effect from its incorporation and augmentation of the synovial membrane and high visco-supplementation properties. The 24 month follow up trial also demonstrated that joint effusion scores decreased significantly over time.
Joints effected by OA typically show joint stiffness, which is a significant source of pain. A recent study on knee joint stiffness in humans supports this concept, showing that the stiffness coefficient was higher in individuals with painful OA. By augmenting with the synovial membrane, which subsequently decreases joint capsule and joint stiffness, the 2.5% PAAG may relieve the pain associated with the OA joint. Indeed experimental studies and clinical trials demonstrate a significant reduction in pain on joint flexion tests in horses diagnosed with OA and treated with 2.5% PAAG.
Summary
The mechanism of action of 2.5% PAAG is likely, that upon injection into joints, the Arthramid® Vet adheres to the synovial lining through its ability to exchange water molecules. This immediately reduces exposure of synoviocytes to pro-inflammatory cytokines in the inflamed or diseased joint. Throughout 14 up to 42 days the gel becomes fully integrated into the synovial lining and its immediate surrounding tissue of the inner capsule by a combination of cell migration and vessel ingrowth forming a thick, cushion-like membrane consisting of vessel integrated gel covered by a new and hypercellular synovial cell lining.
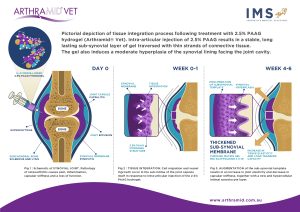
As a result, 2.5% PAAG has a long-lasting augmentation effect on both the joint capsule and synovium. It increases the elasticity and tensile strength of the capsule improving its capacity to transfer load. It is understood that this augmentation and cushioning also causes a subsequent reduction in mechanoreceptor and nociceptor activation in the capsule itself. A reduction in joint cartilage friction and formation of a new and hypercellular synovial cell lining is likely to improve the nature of synovial fluid within the joint itself. These combined actions help reduce the pain of synovitis and restore joint function in the long term.
References
- McIlwraith, C.W., Traumatic arthritis and posttraumatic arthritis in the horse. In: McIlwraith, C.W., Kawcak, C.E., van Weeren, P., editors. Joint disease in the horse. 2nd edition. Elsevier. St Louis, 2016; 2: 33-48.
- Tnibar, A., Persson, A.B., Jensen, H.E., Mechanisms of action of an intraarticular 2.5% polyacrylamide hydrogel (Arthramid Vet) in a goat model of osteoarthritis: Preliminary Observations. SM J Biomed Eng. 2017; 3: 1022.
- Tnibar, A., Schougaard, H., Koene, M., Christensen, L.H., Markussen, B., A controlled clinical trial on the efficacy of an intra-articular polyacrylamide hydrogel in horses with osteoarthritis. 23rd Annual Scientific Meeting of the European College of Veterinary Surgeons (ECVS), Copenhagen, July 2014b.
- De Clifford, L.T., Lowe, J.N., McKellar, C.D., Chambers, M., David, F., A single site, double-blinded, prospective study on the comparative efficacy of a 2.5% polyacrylamide hydrogel in horses with inter-carpal joint lameness. Equine Vet J; [Online] 2019. https://www.sciencedirect.com/science/article/pii/S0737080618307615?dgcid=rss_sd_all
- Tnibar, A., Persson, A., Jensen, H.E., Svalastoga, E., Westrup, U., McEvoy, F., Evaluation of a polyacrylamide hydrogel in the treatment of induced osteoarthritis in a goat model: A pilot randomized controlled Study [abstract].Osteoarthritis Cartilage. 2014; 22: 477.
- Janssen, I., Koene, M., Lischer, C., Intra-articular use of a polyacrylamide hydrogel as a treatment for osteoarthritis in the distal interphalangeal joint: a case series of 12 horses. Pferdeheilkunde. 2012; 28: 650-656.
- Tnibar, A., Persson, A., Jensen, H.E., Svalastoga, E., Westrup, U., McEvoy, F., Evaluation of a polyacrylamide hydrogel in the treatment of induced osteoarthritis in a goat model: A pilot randomized controlled Study [abstract]. Osteoarthritis Cartilage. 2014; 22: 477.
- Henriksen, M., Overgaard, A., Bliddal, H., Initial estimates of efficacy of intra-articular 2.5% polyacrylamide hydrogel for the treatment of knee osteoarthritis: An observational proof-of-concept study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10).
- Narins, R.S. and Schmidt, R., Polyacrylamide hydrogel differences: Getting rid of the confusion. J Drugs Dermatol. 2011; 10(12): 1370-1375.
- Calo, E. and Khutoryanskiy, V.V., Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015; 65: 252-267.
- Christensen, L.H., Breiting, V.B., Aasted, A., Jorgensen, A., Kebuladze, I., Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003; 111(6): 1883-1889.
- Christensen, L., Camitz, L., Illigen, K.E., Hansen, M., Sarvaa, R., Conaghan, P.G., Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthritis Cartilage. 2016; 24: 1999-2002.
- Tnibar, A., Schougaard, H., Camitz, L., Rasmussen, J., Koene, M., Jahn, W., Markussen, B., An international multi-centre prospective study on the efficacy of an intrarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 month follow up. Acta Vet Scand. 2015; 57: 20-27.
- Hall, M.C., Doherty, S., Zhang, W., Doherty, M., Knee stiffness and its relationship to severity of radiographic osteoarthritis, pain and self-reported stiffness. Osteoarthritis Cartilage. 2014; 22: 92.
- Vishwanath, K., Stabler, J., Ziegler, C., and Bonassar, L. Polyacrylamide hydrogel viscosupplements lubricate cartilage after mechanical injury and biochemical degradation. ORS 2021, Annual Meeting Proceedings Paper No. 0685.